Napoleon Monroe
Managing Director, New Directions Technology Consulting
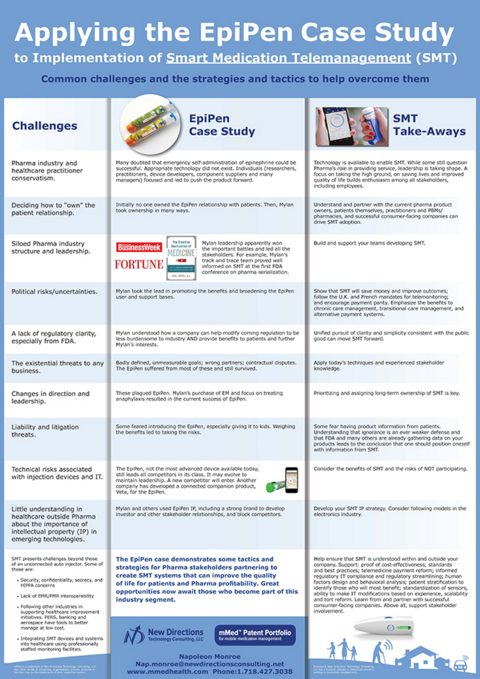
The EpiPen was the groundbreaking civilian auto injector. It is still a notably successful product. There were many barriers to its introduction and eventual success (see recent articles in Fortune and Businessweek). Many are the same barriers for smart medication telemanagement–which can be overcome by using some of the same strategies and tactics that resulted in the EpiPen's success.
Biotech and specialty pharma (often costly, injectable, sensitive products) are the great hope for a better life for many patients. These products are also a focus of many healthcare stakeholders. There are parallels with the EpiPen. Smart medication telemanagement systems allow patients to improve outcomes and provide great value for all stakeholders.
Implementing telemedicine technologies in smart medication telemanagement is especially critical and parenteral product stakeholders have incentives to lead in the efforts.
Despite the fact that there was a known opportunity, there was a perception that EpiPen was just another new complicated thing. Similarly, some still find the concept of providing smart medication management too new. Some want to concentrate only on the same old initiatives used for years. Many of those don't work well in t he changing healthcare environment. Arguably, smart medication telemanagement can help solve many current and coming problems.
Some barriers, issues and threats, as well as observations on possibilities to deal with or overcome them are discussed in this poster to be presented at a session during The Universe of Pre-filled Syringes & Injection Devices conference being held November 3-4, 2015.
View full size case study poster
The EpiPen case demonstrates some tactics and strategies for Pharma stakeholders partnering to create SMT systems that can improve the quality of life for patients and Pharma profitability. Great opportunities now await those who become part of this industry segment.
__________
About Napoleon Monroe
New Directions Technology Consulting Managing Director Napoleon Monroe has an extensive background in developing and producing pharmaceutical delivery systems. His experience also includes managing a Fortune 500 private brand and building/managing the IP portfolio for a company that is now part of Pfizer. His areas of expertise cover product development, manufacturing, licensing, regulatory processes, risk management and international marketing He has experience managing business relationships in more than 30 countries.
Mr. Monroe spent more than two decades at Survival Technology (now a part of Pfizer), where, as a corporate vice president, he was responsible for product development and systems strategy. While there, he led teams that invented, patented and commercialized drug delivery devices, such as the EpiPen, the Antidote Treatment Nerve Agent Auto-Injector delivery system (which still protects U.S. and allied military and civilian personnel), and a number of telemedicine devices.
Mr. Monroe is the sole inventor of the patents in the mMed portfolio.
If you would like more information about this article or about intellectual property for Pharma, please feel free to contact Napoleon Monroe by email at nap.monroe@newdirectionsconsulting.net or visit the mMed Patent Portfolio website at www.mmedhealth.com
________________
mMed is a trademark of New Directions Technology Consulting, LLC.
Any other names of companies, organizations, entities, products or
services may be the trademarks of their respective owners.

No comments:
Post a Comment